Iyini i-titanium dioxide?
Ingxenye eyinhloko ye-titanium dioxide yi-TIO2, okuwumbala obalulekile wamakhemikhali we-inorganic ngendlela yokuqinile okumhlophe noma impushana. Ayinabo ubuthi, inobumhlophe obuphezulu nokukhanya, futhi ithathwa njengebala elimhlophe elingcono kakhulu lokuthuthukisa ubumhlophe bempahla. Isetshenziswa kabanzi ezimbonini ezifana nezingubo, amapulasitiki, irabha, iphepha, uyinki, izitsha zobumba, ingilazi, njll.

Ⅰ.Umdwebo wochungechunge lwemboni ye-Titanium dioxide:
(1)Umfula okhuphukayo wochungechunge lwemboni ye-titanium dioxide uqukethe izinto ezingavuthiwe, okuhlanganisa i-ilmenite, i-titanium concentrate, i-rutile, njll;
(2)I-midstream isho imikhiqizo ye-titanium dioxide.
(3) Ezansi nomfula inkambu yokusetshenziswa kwe-titanium dioxide.I-Titanium dioxide isetshenziswa kabanzi emikhakheni ehlukahlukene efana nezingubo, amapulasitiki, ukwenza amaphepha, uyinki, irabha, njll.

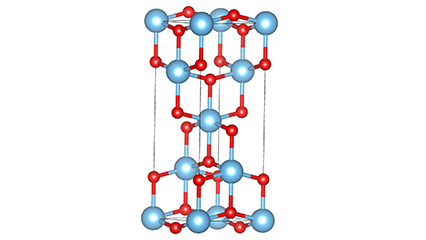
Ⅱ.Isakhiwo sekristalu ye-titanium dioxide:
I-Titanium dioxide iwuhlobo lwenhlanganisela ye-polymorphous, enezinhlobo ezintathu zekristalu ezivamile emvelweni, okuyi-anatase, i-rutile ne-brookite.
Kokubili i-rutile ne-anatase iyingxenye ye-tetragonal crystal system, ezinzile ngaphansi kwezinga lokushisa elivamile; I-brookite ingeye-orthorhombic crystal system, enesakhiwo sekristalu esingazinzile, ngakho-ke inenani elincane elisebenzayo embonini njengamanje.

Phakathi kwezakhiwo ezintathu, isigaba se-rutile yisona esime kakhulu. Isigaba se-Anatase sizoshintsha ngendlela engenakuhlehliswa sibe isigaba se-rutile ngaphezu kuka-900°C, kuyilapho isigaba se-brookite sizoshintsha ngendlela engenakuhlehliswa sibe isigaba se-rutile ngaphezu kuka-650°C.
(1) Isigaba se-Rutile titanium dioxide
Esigabeni se-rutile titanium dioxide, ama-athomu e-Ti atholakala enkabeni ye-crystal lattice, futhi ama-athomu omoya-mpilo ayisithupha atholakala emakhoneni e-titanium-oxygen octahedron. I-octahedron ngayinye ixhunywe kuma-octahedron azungezile angu-10 (okuhlanganisa ama-vertices abelanayo ayisishiyagalombili nemiphetho yokwabelana emibili), futhi ama-molecule amabili e-TiO2 akha iyunithi yeseli.
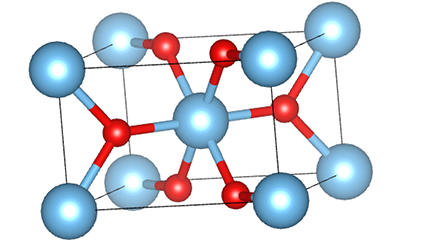
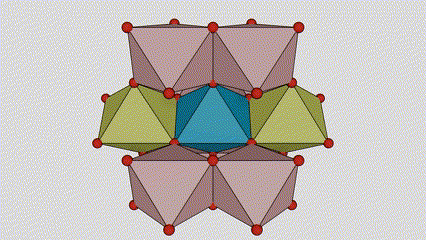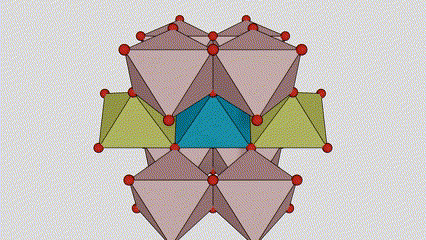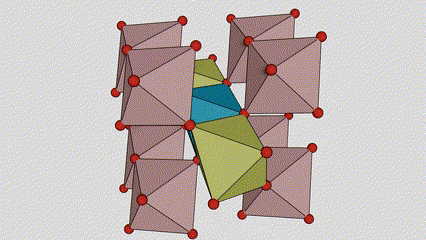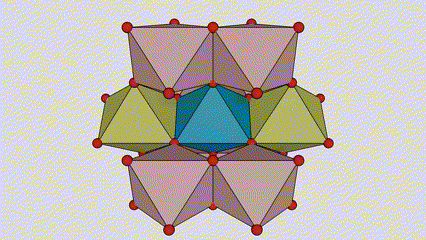
Umdwebo ohleliwe we-crystal cell ye-rutile phase titanium dioxide (kwesokunxele)
Indlela yokuxhuma ye-titanium oxide octahedron (kwesokudla)
(2) isigaba se-Anatase titanium dioxide
Esigabeni se-anatase titanium dioxide, i-titanium-oxygen octahedron ngayinye ixhunywe kuma-octahedron azungezile angu-8 (amaphethelo angu-4 okwabelana kanye nama-vertices angu-4 okwabelana), futhi ama-molecule angu-4 e-TiO2 akha iyunithi yeseli.
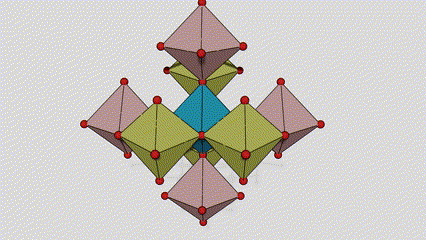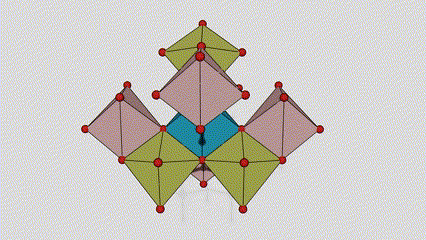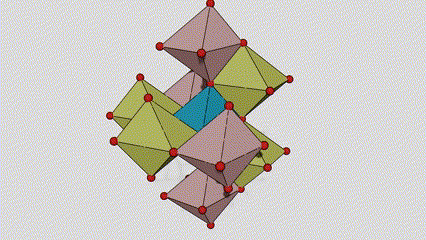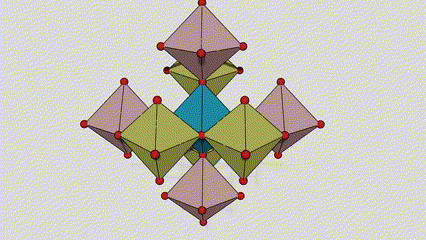
Umdwebo ohleliwe we-crystal cell ye-rutile phase titanium dioxide (kwesokunxele)
Indlela yokuxhuma ye-titanium oxide octahedron (kwesokudla)
Ⅲ.Izindlela Zokulungiselela ze-Titanium Dioxide:
Inqubo yokukhiqiza i-titanium dioxide ikakhulukazi ihlanganisa inqubo ye-sulfuric acid kanye nenqubo ye-chlorination.

(1) Inqubo ye-Sulphuric acid
Inqubo ye-sulfuric acid yokukhiqizwa kwe-titanium dioxide ihilela ukusabela kwe-acidolysis ye-titanium iron powder ne-concentrated sulfuric acid ukukhiqiza i-titanium sulfate, esuke i-hydrolyzed ukuze ikhiqize i-metatitanic acid. Ngemuva kokubala nokuchotshozwa, kutholakala imikhiqizo ye-titanium dioxide. Le ndlela ingakhiqiza i-anatase ne-rutile titanium dioxide.
(2) Inqubo ye-Chlorination
Inqubo ye-chlorination yokukhiqizwa kwe-titanium dioxide ihilela ukuxuba i-rutile noma i-high-titanium slag powder ne-coke bese kwenziwa i-chlorination ephezulu lokushisa ukukhiqiza i-titanium tetrachloride. Ngemuva kwe-oxidation yezinga eliphezulu lokushisa, umkhiqizo we-titanium dioxide utholakala ngokuhlunga, ukuwashwa kwamanzi, ukomiswa, nokuchotshozwa. Inqubo ye-chlorination yokukhiqizwa kwe-titanium dioxide ingakhiqiza kuphela imikhiqizo ye-rutile.
Ungahlukanisa kanjani ubuqiniso be-titanium dioxide?
I. Izindlela Zomzimba:
(1)Indlela elula ukuqhathanisa ukuthungwa ngokuthinta. I-titanium dioxide mbumbulu izwakala ishelela, kuyilapho i-titanium dioxide yangempela izwakala ilukhuni.

(2)Ngokugeza ngamanzi, uma ufaka i-titanium dioxide esandleni sakho, ewumgunyathi kulula ukuyigeza, kuyilapho engokoqobo akulula ukuyigeza.

(3)Thatha inkomishi yamanzi ahlanzekile bese uphonsa i-titanium dioxide kuyo. Le entanta phezulu ingeyeqiniso, kuyilapho leyo ehlala phansi iyimbumbulu (le ndlela ingase ingasebenzi emikhiqizweni ecushiwe noma eshintshiwe).
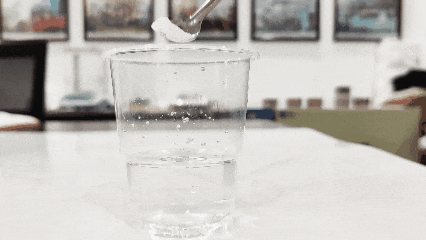

(4)Hlola ukunyibilika kwayo emanzini. Ngokuvamile, i-titanium dioxide iyancibilika emanzini (ngaphandle kwe-titanium dioxide eklanyelwe ngokukhethekile amapulasitiki, oyinki, kanye ne-synthetic titanium dioxide, engancibiliki emanzini).

II. Izindlela zamakhemikhali:
(1) Uma i-calcium powder yengezwa: Ukwengeza i-hydrochloric acid kuzobangela ukusabela okunamandla ngomsindo ohlabayo, okuhambisana nokukhiqizwa kwenani elikhulu lama-bubbles (ngoba i-calcium carbonate isabela nge-asidi ukuze ikhiqize i-carbon dioxide).

(2) Uma i-lithopone yengezwa: Ukwengeza i-dilute sulfuric acid noma i-hydrochloric acid kuzoveza iphunga leqanda elibolile.

(3) Uma isampula i-hydrophobic, ukwengeza i-hydrochloric acid ngeke kubangele ukusabela. Kodwa-ke, ngemva kokuyimanzisa nge-ethanol bese wengeza i-hydrochloric acid, uma amabhamuza ekhiqizwa, kufakazela ukuthi isampula iqukethe i-calcium carbonate powder ehlanganisiwe.

III. Kukhona nezinye izindlela ezimbili ezinhle:
(1) Ngokusebenzisa ifomula efanayo ye-PP + 30% GF + 5% PP-G-MAH + 0.5% titanium dioxide powder, amandla aphansi ezinto eziwumphumela, i-titanium dioxide (rutile) iyiqiniso kakhulu.
(2) Khetha inhlaka esobala, njenge-ABS esobala ene-0.5% titanium dioxide powder eyengeziwe. Linganisa ukukhanya kwayo. Lapho ukukhanya kungaphansi, yilapho i-titanium dioxide powder iyiqiniso kakhulu.
Isikhathi sokuthumela: May-31-2024





